Patented delivery technology in a lotion2,3


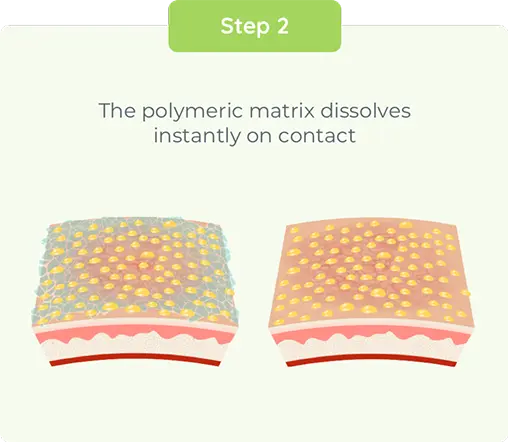



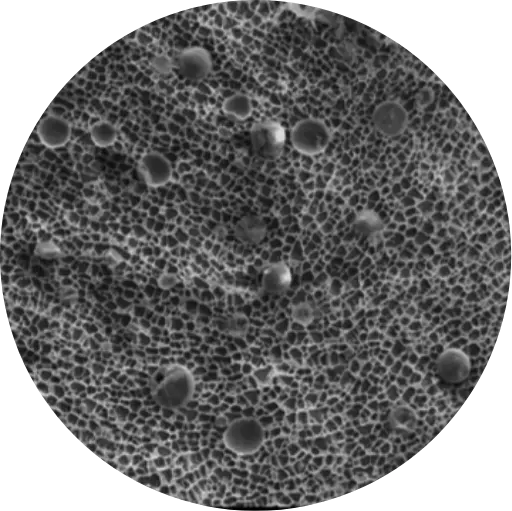
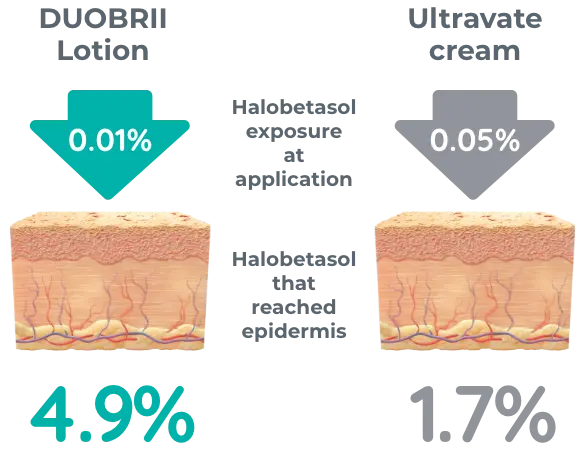
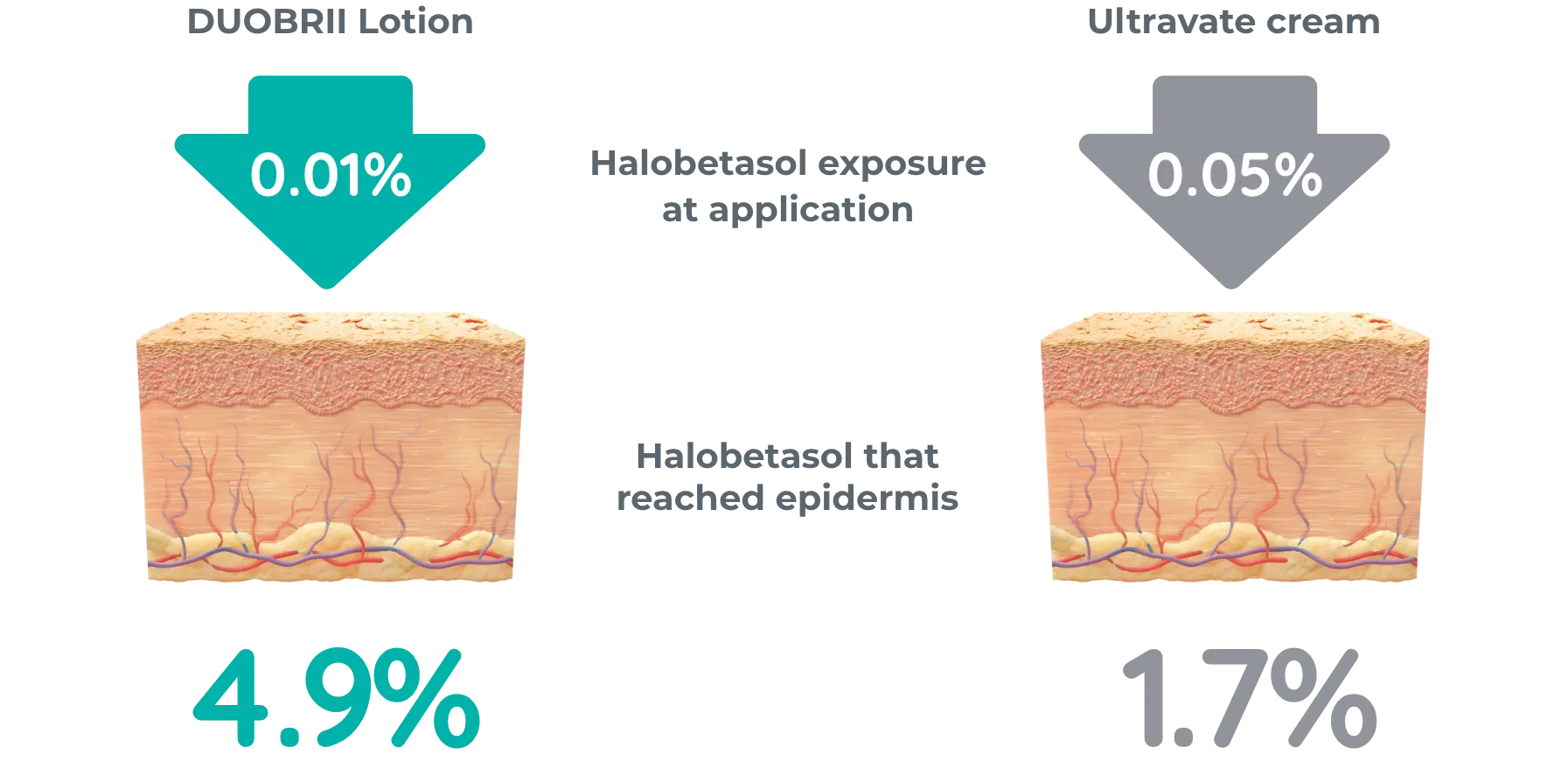
Ex vivo study vs Ultravate cream of 20 skin grafts from cadaver subject
DUOBRII Lotion delivery technology—results after single application1,2
DUOBRII Lotion is contraindicated in pregnancy.
To report SUSPECTED ADVERSE REACTIONS, contact Ortho Dermatologics at 1-800-321-4576 or FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
DUOBRII® (halobetasol propionate and tazarotene) Lotion, 0.01%/0.045%, is indicated for the topical treatment of plaque psoriasis in adults.
Please click here for full Prescribing Information.
References: 1. Ozyurekoglu E, Kircik LH. An open-label pilot study to investigate safety and efficacy of fixed combination tazarotene 0.045% and halobetasol propionate 0.01% lotion for the treatment of scalp psoriasis. J Drugs Dermatol. 2021;20(11):1191-1194. 2. Tanghetti EA, Gold LS, Del Rosso JQ, et al. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J Dermatolog Treat. 2019;1-8. DOI: 10.1080/09546634.2019.1668907. 3. Data on file.
DUOBRII Lotion is contraindicated in pregnancy.
DUOBRII Lotion is contraindicated in pregnancy.